FROM EDITOR
PHARMACOECONOMICS
The effective control of Diabetes Mellitus (DM) is an actual problem from optimal expenditures of health care system point of view. Agonists of receptors for glucagon like peptide-1 (aGLP-1) are one of the modern option for glycemia control in DM Type 2 and included in all current guidelines for the treatment control. The economic comparative aspects of the use of these drugs in the local conditions have not been studied. Materials and methods. Comparative economic evaluation of insulin glargine + lixisenatide (iGlaLixi), exenatide (Exe), dulaglutide (Dula), liraglutide (Lira) and combinations of their aGLP-1 with iGla 100 U has been performed base on published clinical data of efficacy. Number of patients with HbA1c <7 % was chosen as efficacy criterion. Non-direct comparison with Odds Ratio (OR) calculation was prepared. Direct and indirect costs (medications, treatment of CV-complications, GDP loses etc.) were indicated and calculated based on constructed model. Sensitivity analysis has been provided for validation of results. Results. Pharmacoeconomic analysis based on non-direct efficacy comparisons of iGlaLixi, Exe, Lira and Dula has shown of economic advantages of effective DM2T control. iGlaLixi has demonstrated economic advantages as well usage aGLP-1 only (direct costs decreasing vs Exe on 23,8 %, vs Dula on 15,6 %, vs Lira on 54,4 %) as their combinations with iGla 100 U (direct costs decreasing vs iGla 100 U + Exe on 23,8 %, vs iGla 100 U + Dula on 15,2 %). iGlaLixi decreased a total cost (direct and non-direct) better than Exe, Dula and Lira (on 19,9, 9,3 и 45,2 % accordingly). Conclusion. An effective control of DM2T with aGLP-1 and medicines on their base has an economic value because lead to expenditures for complications decreasing from government position.
Relevance. A viral pandemic caused by the SARS-CoV-2 coronavirus has led to the development of a new coronavirus disease-2019 (COVID-19). The COVID-19 pandemic has forced the mobilization of all available health system resources. There are separate publications on reducing the risk of developing coronavirus infection in people vaccinated against influenza. Objective: to study the cost-effectiveness of influenza vaccination in the conditions of the» first « wave of COVID-19. Materials and methods. The archival data of 2,452 people from among the sick employees of JSC «Russian Railways» were analyzed. The control group consisted of 2,911 employees who were not infected with COVID-19, comparable by gender, age and territory of residence. Scores on the Charlson comorbidity scale were calculated for all individuals. The pharmacoeconomical cost of the patient’s treatment was predicted using the Markov model. Results. Having a flu shot reduced the likelihood of getting COVID-19 by 1.3 times. In the presence of a diagnosis of coronavirus infection, inpatient treatment for influenza vaccinated patients was required 2 times less often than for unvaccinated patients. Compared to the situation of the absence of vaccinated persons, in the «first wave», the estimated cost savings for the treatment of patients with coronavirus infection amounted to 124 million rubles. When the number of points on the comorbidity scale increased from 1 to 8, the average cost of treatment of patients without previous influenza vaccination increased by 2 times, and in the presence of vaccination, the average cost of treatment increased by 1.7 times. Conclusion. Thus, this study shows that influenza vaccination is cost-effective against COVID-19. The effect is achieved by reducing the likelihood of getting a coronavirus infection in the presence of a flu shot.
Objective: to review the data on the efficacy and consumption of octocog alfa and rurioctoctog alfa pegol in standard prophylaxis and individualized prophylaxis in hemophilia A patients based on published international data. Material and methods: a systematic literature search and review were performed. Among 25 sources identified within the systematic search 7 relevant sources describing the comparison of treatment with octocog alfa and rurioctocog alfa pegol in adult and pediatric patients with severe and moderate hemophilia A based on personalized assessment of the pharmacokinetic curve using the interactive tool myPKFit versus the standard (non-personalized) dosage regimen were selected. Data on individual patients, as well as data from secondary subgroups defined by age, bleeding rate, risk of bleeding associated with the daily physical activity were combined and analyzed. Results. In observational studies, adjustments of the dose and administration of octocog alfa in patients with severe hemophilia based on personalized assessment of the pharmacokinetic curve using myPKFit resulted in the reduced consumption and/or increased efficacy of prophylaxis — a reduced annual bleeding rate. In an extended controlled study of rurioctocog alpha pegol a trend toward reduced bleeding rate and increased mean annual consumption of the drug was reported in patients who received myPKFit guided prophylaxis compared to a non-personalized treatment regimen. In the single-cut studies, myPKFiT use resulted in the regimen revisions in less than a quarter of patients. Summary. Personalized dosing for octocog alpha and rurioctocog alpha pegol based on pharmacokinetic curve built using pharmacokinetic population model enables reasonable dose adjustments and improves outcomes.
PHARMACOEPIDEMIOLOGY
Currently bacterial community-acquired pneumonia (CAP) remains one of the important problems of providing medical care on an outpatient basis. Despite the high detection rate and modern methods of treatment this disease holds the first place among the causes of death in the category of infectious diseases. Knowledge about rational use of drugs is obtained in higher medical school and subsequently serves as a basis for further work of a practicing physician. Purpose of the study: to analyze the knowledge of final year medical students in the field of rational choice of antimicrobial agent (AMA) in the treatment of non-severe CAP in outpatient setting in patient without concomitant diseases and risk factors. Total 240 final year students of A.I. Yevdokimov Moscow State University of Medicine and Dentistry were offered in February-April, 2019 to indicate their preferred AMA for a young previously healthy patient with mild CAP. The study involved 178 women (74.17 %) and 62 men (25.83 %). The average age of the respondents was 24.8+3.3 years. There were 271 options for the appointment of AMA with 152 (56.1 %) given by the international nonproprietary name and 119 (43.9 %) by trade name. Remarkably, the AMAs which are recommended for the treatment of mild CAP on an outpatient basis, accounted only for 46.2 % in this study. Of particular concern is the fact that only about 40—50 % of AMA prescribing for CAP by medical graduates is in line with current clinical guidelines. The inappropriate choice of a drug in this particular situation not just increases the drug load, the cost of pharmacotherapy and the risk of side effects, but also directly affects the results of treatment. This situation emphasizes the need for a purposeful formation of a personal formulary of medicines for a graduate of a medical university, taking into account the basic principles of rational pharmacotherapy and the provisions of clinical guidelines relevant to the Russian Federation.
DRUG SAFETY
Interstitial lesion is one of the causes of respiratory failure. Drugs are a modifiable etiological factor of lung damage. Medications most commonly associated with drug-induced interstitial lung disease include antineoplastic drugs, disease-modifying anti-rheumatic drugs and amiodarone. According to the latest literature data, the previously described link between anti-rheumatic drugs and interstitial lung diseases is very inconsistent. It may even be a protective effect of this group of drugs on the lung tissue. The relationship between statin use and interstitial lesions is also complex and not fully understood. It is necessary to carefully assess the appearance of respiratory tract complaints in patients taking statins as in other groups of patients. Prescription of additional diagnostic methods is necessary to close monitoring and prevention the toxic effect of these drugs. These actions, as well as the potential prescription of steroid therapy and change in the underlying disease treatment approaches, are an important factor in reducing the incidence of respiratory failure in the population.
Administration of a rational and safe drug therapy is one of the most challenging issues for healthcare professionals. The frequency of hospitalizations due to the adverse drug reactions in the years 2000 — 2015 was estimated at 6.3 (3.3—11.0 %) for developed countries and 5.5 % (1.1—16.9 %) for developing countries. It is known that alcohol intake is a risk factor for many socially significant diseases, including arterial hypertension, coronary heart disease, chronic heart failure, etc., however, many doctors pay insufficient attention to the fact that many drugs, for example, beta-blockers, antidepressants, bezodisepines, calcium antagonists, can interact with alcohol when consumed simultaneously and, thus, increase the risks of adverse drug reactions. There are 2 main types of interactions between alcohol and drugs: pharmacokinetic (at the stage of absorption, distribution, metabolism and elimination) and pharmacodynamic (at the stage of effects and receptors). For example: the simultaneous intake of alcohol and paracetamol leads to the formation of toxic metabolites due to the induction of cytochrome P450 isoenzymes by alcohol. Another example is decrease in presystemic elimination and stimulation of the metabolism of tricyclic antidepressants; an increase in the elimination of imipramine and desipramine in patients with chronic alcoholism after detoxification therapy, and so on. In this article, the authors analyzed and systematized data from open literature sources in order to inform health care professionals about the possible risks associated with the interaction of alcohol and drugs and various pharmacological groups.
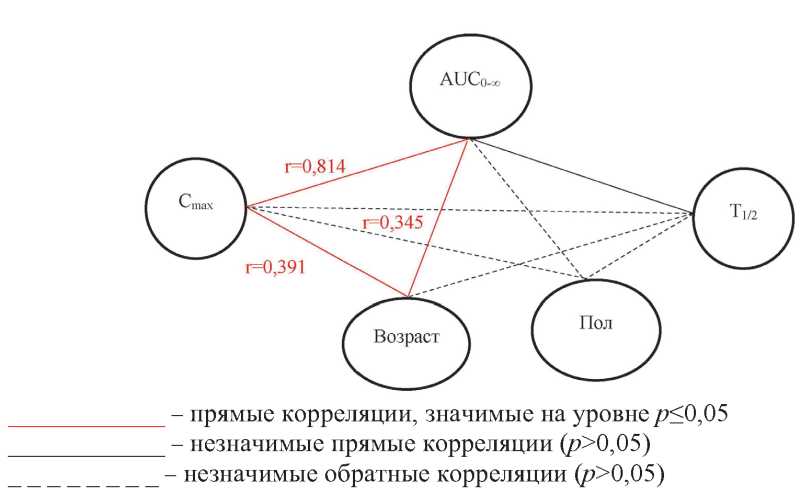
PHARMACOKINETICS
Fludarabine is a purine antimetabolite with a pronounced immunosuppressive effect. The inhibitory effect of fludarabine depends on its concentration in blood plasma. In addition, the phenotypic characteristics of patients affect the pharmacokinetic and pharmacodynamic profile of the drug, which necessitates a personalized approach to the dosage regimen. The chromatography-mass spectrometric method for the quantitative determination of 2-fluorine in blood plasma was developed for studying the individual parameters of pharmacokinetics of the international non-proprietary name (INN) fludarabine in patients with B-cell chronic lymphocytic leukemia during the standard course. Such method for the quantitative determination of 2-fluorine in blood plasma was developed and validated in accordance with international requirements. Significant individual variability of the main pharmacokinetic parameters in patients with B-cell chronic lymphocytic leukemia with a single oral administration of the drug with INN fludarabine at a dose of 40 mg/m2 was established, so the coefficient of variability Cmax was 42 %, Tmax — 92 %, AUC0-t — 45 %, Kel — 23 %, T1/2 — 26 %. It should be noted that there is a high interindividual variability of fludarabine, for example, 24 hours after taking the study drug, the maximum and minimum plasma concentrations of the fludarabine metabolite 2-fluoro-ara-A in different in patients with B-cell chronic lymphocytic leukemia differed 9 times. Individual variability of pharmacokinetic parameters characterizing absorption (Cmax/AUC0-t) and total clearance of the active metabolite of fludarabine is statistically significantly associated with a combination of gender and anthropometric factors.
PHARMACOGENETICS
Introduction. Antipsychotics are the main drugs for treatment of schizophrenia spectrum disorders. Pharmacodynamic genetic factors are being actively studied to improve the accuracy of antipsychotic selection based on pharmacogenetic testing. Purpose of this study: to establish associations of genetic polymorphisms of the DRD2, DRD3, DRD4, HTR2A, COMT, ZNF804A, and ANKS1B genes with the efficacy and safety of antipsychotics in adolescents with an acute psychotic episode during a 28-day follow-up. Materials and methods. The study included 68 adolescents with an established diagnosis of acute polymorphic psychotic disorder at the time of admission (F23.0-9 according to ICD-10). All patients received an antipsychotic as their main therapy. Patients were monitored for 28 days. The effectiveness of antipsychotics was assessed using the Children’s Global Assessment Scale (CGAS), Positive and Negative Symptoms Scale (PANSS), Clinical Global Impression Severity (CGI-S) and Improvement (CGI-I). The safety of pharmacotherapy was assessed using the UKU Side Effects Rating Scale (UKU SERS), Sympson-Angus Scale (SAS), Barnes Akathisia rating scale (BARS). From each patient we obtained a buccal scraped epithelium, extracted DNA from it by sorbent method and detected carriage of genetic polymorphisms DRD2 rs1800497 (C>T), DRD3 rs6280 (C>T), DRD3 rs324026 (C>T), DRD4 rs1800955 (C>T), HTR2A rs6313 (T>C), COMT rs4680 (Val158Met, G>A), ZNF804A rs1344706 (A>C), ANKS1B rs7968606 (C>T) by real-time PCR. Results. DRD2 rs1800497 T allele carriers had a stronger reduction in the PANSS subscore «Productive Symptomatics» on day 14 (Me=-7.0 [-9.0;-5.0] vs Me=-7.0 [-8.0;-2.0]; p=0.018) and day 28 of follow-up (Me=-11.0 [-9.0;-5.5] vs Me=-8.0 [-8.0;-2.0]; p=0.019). Also, greater improvement on the CGAS scale on day 14 of follow-up was seen in TC+CC HTR2A rs6313 carriers (Me=2.0 [1.0;3.0] vs. Me=2.0 [1.0;2.0]; p=0.029). DRD3 rs324026 homozygous carriers (TT) had a significantly lower SAS score (Me=0.5 [0.0; 1.0] vs. Me=1.0 [0.0; 5.0]; p=0.016) and UKU subscore «Neurological Disorders» on 28 days of antipsychotic therapy (Me=0.0 [0.0; 0.0] vs. Me=1.0 [0.0; 3.8]; p=0.005). DRD3 rs324026 TT carriers also had lower severity of akathisia according to the BARS scale. Carriers of the T DRD4 rs1800955 allele had a higher SAS scale score on day 28 of therapy compared with CC homozygotes (Me=1.0 [0.0;4.0] vs Me=0.0 [0.0;1.0]; p=0.036). Conclusion. The DRD2 rs1800497 was a predictor of better reduction of productive symptoms; HTR2A rs6313 demonstrated a similar association. The DRD2 rs1800497 polymorphic variant was a predictor of better reduction of productive symptomatology; HTR2A rs6313 demonstrated a similar association. DRD3 rs324026 and HTR2A rs6313 were associated with a lower frequency of neurological adverse reactions and akathisia. In contrast, carriers of the DRD4 rs1800955 were more prone to adverse reactions on pharmacotherapy.
BIOMEDICAL ETHICS
This article outlines bioethical issues related to the application of the Internet of Body (IoB) technology in health care so-called medical IoB devices. Manufacturers of medical IoB devices promise to provide significant health benefits, improved treatment outcomes and other benefits, but such IoB also carry serious risks to health and life, including the risks of hacking (cyberhacking), malfunctioning, receiving false positive measurements, breaching privacy, deliberate invasion of privacy. In addition, medical IoB products can directly cause physical harm to the human body. As human flesh is intertwined with hardware, software, and algorithms, the IoB will test our social values and ethics. In particular, IoB will challenge notions of human autonomy and self-government as they threaten to undermine the fundamental precondition of human autonomy. Thus, the protection of human autonomy should become the main ethical principle of the use of medical IoB devices.
ISSN 2618-8473 (Online)