FROM EDITOR
CLINICAL PHARMACOLOGY
The novel coronavirus infection (COVID-19) is characterized by damage, along with the lungs, to many other vital organs and systems. The prevalence and severity of the resulting lesions are determined by the ability of the SARS-CoV-2 virus to cause excessive activation of the immune response, accompanied by changes in both cellular and humoral components. Among humoral disorders, the most significant is the hypersecretion of cytokines, including interleukins (IL), in particular IL-6 and IL1β. Elevated levels of IL-6 are one of the main predictors of severe COVID-19 and death. So, blocking the effects of this cytokine is of fundamental importance for improving the clinical outcomes of patients. Monoclonal antibodies against human interleukin-6 receptor or against IL-6 have been widely studied in patients with extremely severe COVID-19, and to a lesser extent in mild and moderate severity. Regardless of the severity, maximum effectiveness is achieved when these drugs are administered as early as possible, which made it possible to create such a tool as preventive anti-inflammatory therapy. Preventive prescription of IL-6 inhibitors may be useful not only for hospitalized inpatients, but also for outpatients. This review is aimed to assess the effectiveness of early use of IL-6 inhibitors both in hospitalized and ambulatory patients with varying degrees of severity of COVID-19.
NEW HEALTH TECHNOLOGY
Relevance. The SARS-CoV-2 (COVID-19) pandemic highlighted the use of telemedicine in healthcare delivery during a public health emergency to remotely assess and provide care to patients already infected with the coronavirus or those fearing exposure to COVID-19 through face-to-face contact.
Objective. Explore the response of the world’s health systems and their medical institutions to the situation caused by the COVID-19 pandemic using telemedicine and its practical application and contribution to health care in the era of the COVID-19 pandemic.
Methods. A search analysis of published literature data was conducted using the English-language database of medical and biological publications PubMed. The most up-to-date information is obtained from relevant websites.
Results. The concept of telemedicine, its legislative framework, and current trends in its practical application are discussed. It has been confirmed that there has been a sharp increase in the use of telemedicine and mobile applications in response to the COVID-19 pandemic and the need for infection control. Assessed healthcare provider, health insurance, and pharmacy policy reforms in several countries to integrate telemedicine into practice in response to the COVID-19 pandemic. It has been established that the expansion of the use of telemedicine in the COVID-19 era has confronted society as a whole with the need to solve some practical problems that impede progress in this area. The recommendations of professional medical organizations regarding telemedicine during the COVID-19 pandemic were analyzed, and attempts were made to solve the problems of advanced telemedicine practice.
Conclusion. The use of telemedicine is undeniably effective in promoting social distancing and isolation/quarantine of patients, which is a proven means of strengthening the public health position in countering the spread of coronavirus. The need for social distancing has made telemedicine a critical factor in the fight against the pandemic spread of COVID-19.
DRUG SAFETY
The Global Trigger Tool is a reliable method for detecting adverse events, demonstrating positive predictive value and significant sensitivity among patients with COVID-19.
The objective of this study was to develop and apply an adapted Global Trigger Tool to identify potential adverse events among hospitalized patients with moderate to severe COVID-19.
Materials and methods. The study included a literature review, analysis of spontaneous reports n=873 in the national database of the Russian Federation for the COVID-19 indication for the period 2020–2022, and application of the trigger tool in a single-center retrospective study n=329. Statistical processing was performed by the method of disproportionality with the determination of the odds ratio of reporting. The symptom-syndrome method based on parameterization of interaction effects using Zhegalkin polynomials was used to identify significant effects of drug associations; Fisher’s exact test was used to select the most significant associations for occurrence.
Results. Among the triggers with a frequency of 10 or higher in terms of mortality rate (≥80%), the leading ones were: pulmonary edema, hypotension, and respiratory failure, which is consistent with the COVID-19 clinical course of the disease. A disproportionately high incidence of respiratory failure was associated with favipiravir use. Leukocytosis associated with tofacitinib use and drug-induced liver damage associated with tocilizumab use.
Conclusions. The identified potential adverse events and their associated mortality risks among patients with moderate to severe COVID-19 allow for compliance with current clinical guidelines for the management of patients with COVID-19.
Relevance. Chronic kidney disease (CKD) is often concomitant pathology in patients with atrial fibrillation (AF). Because of the increased risk of bleeding in patients with AF and CKD while taking anticoagulant therapy, it is necessary to assess new methods for predicting the risk of bleeding when prescribing anticoagulants in this category of patients.
Objective. To evaluate the possible relationship between the presence of bleeding in patients with AF and CKD C3–4 receiving rivaroxaban and the level of renal damage markers in urine.
Methods. One hundred and thirty-three patients with AF and CKD C3a-C4 aged 52 to 97 years (median age 82 [74;86] years) were included in the study. All patients were assessed for bleeding and excretion of markers of renal damage (albumin; nephrin; neutrophil gelatinase-associated lipocalin (NGAL), and kidney injury molecule-1 (KIM-1)) in with urine have been identified. In addition, the levels of kidney injury markers in the urine of 45 healthy volunteers were analyzed.
Results. Urinary NGAL and KIM-1 levels in patients with AF and CKD with a history of bleeding (5.5 [3.81;23.83] ng/ml and 0.68 [0.27;1.10] ng/ml, respectively) were significantly higher than those in patients without bleeding (4.19 [2.22; 15.53] ng/ml, p=0.039, and 0.38 [0.13;0.66] ng/ml, p=0.019, respectively) and healthy subjects (2.6 [1.9;4.3] ng/ml, p<0.001, and 0.21 [0.10;0.69], p=0.003, respectively).
Conclusion. Patients with AF and CKD C3a — C4 on rivaroxaban treatment with a history of bleeding have higher urinary excretion of KIM-1 and NGAL.
DRUGS THERAPEUTIC MONITORING
Relevance. Vancomycin, like other glycopeptide antibiotics, is characterized by its pharmacokinetic and pharmacodynamic profile by both time-dependent and concentration-dependent bactericidal effect. Its optimal achievement is ensured by maintaining the drug concentration several times higher than the minimum inhibitory concentration (MIC). Exceeding peak concentrations of vancomycin (> 20 µg/ml) increase the risk of nephrotoxicity, and extremely low (<9.9 µg/ml) — selection of resistant forms of Gram-positive microorganisms.
Objective of this study is to evaluate the achievement of target level of vancomycin concentration in plasma of patients with deep suppuration after hip arthroplasty based on therapeutic drug monitoring.
Methods. The study included patients who received infusion therapy with vancomycin in the purulent department of traumatology and orthopedics of the University Clinic of the Privolzhsky Research Medical University for deep suppuration after hip arthroplasty in the period from 01.03.2023 to 30.06.2023. The study was conducted without correction for the trade name of vancomycin. Therapeutic drug monitoring was performed on the third day after the start of therapy (after the 4th administration). Blood sampling was performed 1 hour after infusion and 1 hour before subsequent infusion. Vancomycin in blood plasma was determined by high-performance liquid chromatography using a chromatograph "LC-20 Prominance" (Shimadzu, Japan) in reverse phase mode with matrix photodiode detector of UV and visible spectra (SPD–M20A).
Results. A total of 14 patients were included in the study, including 6 males and 8 females. The mean age of the patients was 60.36±12.38 years. Bacterial flora was detected in all patients included in the study. Resistant Gram-positive microorganisms were isolated: St. aureus (MRSA) — 5, in 9 patients — coagulase-negative staphylococci (St. epidermidis (MRSE) — 7, St. simulans — 2). Therapeutic concentrations of both initial and residual concentrations were achieved in 28.57% of cases. In 71.43% of cases residual concentrations had values <10 µg/ml, which corresponded to extremely low values, not sufficient for clinical effect associated with eradication of the pathogen at MPC = 1 µg/ml. At the same time initial concentrations of vancomycin were defined as extremely low in 14.29% of cases, and in 42.86% — as exceeding the therapeutic range. Vancomycin concentrations in the therapeutic range of 10 to 20 µg/ml 1 hour after infusion (initial concentrations) were determined in 42.86% of patients.
Conclusion. The results of therapeutic drug monitoring of vancomycin in patients with deep suppuration after hip arthroplasty show a wide range of concentrations. A high proportion of residual concentrations at extremely low levels (<9.9 μg/ml) was observed, which is consistent with the results of other studies and confirms the need for therapeutic drug monitoring in every patient receiving vancomycin therapy.
CLINICAL TRIALS
Actuality. The speed of patient recruitment into a clinical trial allows us to evaluate the work of the clinical center and calculate the time required to achieve targeted recruitment. The authors estimate the recruitment rate on the basis of comparison with the target recruitment value, and according to the literature, the recruitment rate is influenced by many factors, which mainly have a negative effect, reducing it. Assessments of the optimality of recruitment or the normality of recruitment of patients according to the literature were always made based on a specific protocol and nosology because this parameter cannot be constructed experimentally under ideal conditions in the absence of the influence of one or another factor. On the other hand, most clinical trials fail to recruit patients, and accordingly, the recruitment rate in such studies was strongly influenced by certain factors. For the first time, we assessed the patient recruitment rate in successful studies when calculating the degree of influence of the nosology and found the ideal recruitment rate, i. e., the recruitment rate with zero influence of the factor.
Objective. To quantify the degree of influence of the nosology factor of the protocol on the recruitment rate of patients in a clinical trial.
Materials and methods. A retrospective analysis of 4 international multicenter clinical trials of II–III phases was conducted on the recruitment of patients depending on the influence of the nosology factor of the protocol. Descriptive statistics using the typing and odds ratio technique.
Results and discussion. A quantitative assessment of the influence of the nosology factor of the protocol on the rate of patient recruitment was obtained. Found the ideal typing speed.
Conclusions. For the first time, a quantitative assessment of factors influencing patient recruitment has been proposed. For the first time, the ideal recruitment rate has been determined.
DRUGS UTILIZATION RESEARCH
Relevance. Colon and ovarian cancer occupy leading positions in the structure of morbidity and mortality among malignant neoplasms in Russia. One of the widely used drugs for the treatment of these nosologies is oxaliplatin, but there is no relevant information to assess its availability and share among other antitumor drugs.
Objective. Analysis of the Russian pharmaceutical market for oxaliplatin drugs among other antitumor drugs in 2015–2022.
Materials and methods. For the analysis, reports from marketing agencies DSM Group and IMS were used, both in value and in physical terms.
Results. During the period under study, significant changes occurred in the Russian pharmaceutical market of antitumor drugs: the volume of sales and consumption increased, which was also accompanied by a decrease in the cost of drug units per package (all antitumor drugs) and course of use (oxaliplatin). At the same time, the share of Russian manufacturers of antitumor drugs has increased. In relation to oxaliplatin, during the study period there was almost complete displacement of the original drug from the market by generic drugs. An intensive process of import substitution was also observed: the share of domestic drugs increased by the end of the study period in value and volume terms to 93 and 95%, respectively.
Conclusion. Identified changes in the Russian pharmacological market for 2015–2022 reflect an increase accompanied by an increase in the availability of antitumor drugs and, in particular, oxaliplatin.
EXPERT OPINIONS
The advisory board was held to discuss he current situation with diagnosis and treatment of advanced non — small cell lung cancer with a mutation in the MET gene (aNSCLC with METex14). Lung cancer takes a leading place in the structure of oncological diseases in Russia. The frequency of molecular abnormalities in the MET gene patients with advanced lung cancer is 3%, the NGS method is the most effective in identifying this alteration.
The effectiveness of capmatinib, a MET tyrosine kinase inhibitor was confirmed in the GEOMETRY mono-1 registry trial: the objective response rate in the patients with aNSCLC with METex14 who had previously received 1–2 lines of therapy was 41%, among those who had not previously received treatment — 68%. Median duration of response was 9.7 month in previously treated patients with aNSCLC with METex14 and 12.6 months in the treatment — naïve patients with aNSCLC with METex14. Intracranial response was observed in 54% of cases among patients with aNSCLC with METex14. In addition, the efficacy of capmatinib was confirmed when it is administered to the patients with aNSCLC with METex14 in real-world clinical practice. Other therapeutic options (immune-oncology therapy, chemotherapy) are less effective in case of this disease variant.
Based on the data on effectiveness and tolerability of capmatinib, the expert group made a decision to recommend inclusion of capmatinib in the Russian Clinical Guidelines for the treatment of lung cancer and into the Essential Drug list.
INTERNAL MEDICINE
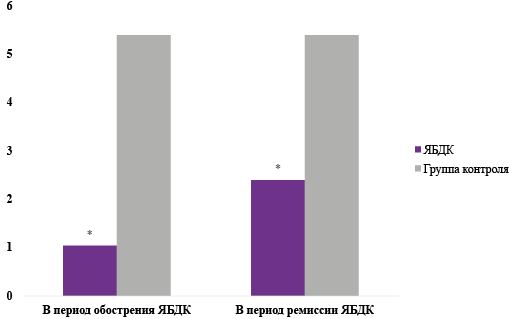
The purpose. To study the content of melatonin in venous blood, to assess the seasonal variability of the hormone in patients with duodenal ulcer (DU) and to establish a possible relationship between the studied parameters.
Material and methods. 45 patients with varying clinical activity of DU during exacerbation and during treatment were examined. Determination of melatonin in venous blood was carried out using high-performance liquid chromatography with tandem mass spectrometry. To assess the condition of the duodenum, fibrogastroduodenoscopy of the gastroduodenal zone we used Exera (cIF160) and Olympus endoscopes.
Results. It has been established that in the active stage of DU the levels of the hormone melatonin are reduced. In order to study the possible effect of light illumination on melatonin content, we studied the production of this indole in groups of patients with DU at different times of the year. It turned out that the melatonin content was significantly lower in the group of patients with peptic ulcer disease in winter and spring.
Conclusion. The regression and correlation analysis carried out revealed a positive moderate dependence of the melatonin indicator on the duration of daylight hours in the venous blood. Clinical remission of DU after 2 months was accompanied by an increase in melatonin production.
OBITUARY
ISSN 2618-8473 (Online)